The Future Of Cancer Care Is Glowing: LED-Driven Nanoflakes Bring New Hope To Patients
A Brighter Future for Cancer Treatment
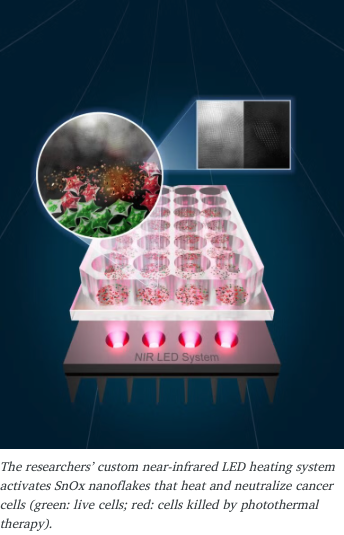
Researchers at The University of Texas at Austin and the University of Porto have unveiled a revolutionary light-based cancer therapy that could change how we treat cancer, including breast cancer, in the future. Using gentle LED lightand ultra-thin tin nanoflakes (SnOx), this innovative treatment destroys cancer cells while keeping healthy tissue safe.
Unlike chemotherapy or radiation, which often cause painful side effects, this new method is non-invasive and far gentler on the body. By harnessing the precision of near-infrared light, scientists are bringing hope for more targeted, affordable, and accessible cancer care.
How It Works: Light Meets Science
The team designed a custom near-infrared LED system that activates the SnOx nanoflakes, heating them just enough to neutralize cancer cells, without harming surrounding healthy cells.
“Our goal was to create a treatment that is not only effective but also safe and accessible,” said Jean Anne Incorvia, professor at UT Austin’s Cockrell School of Engineering. “With LED light and SnOx nanoflakes, we can precisely target cancer cells while leaving healthy tissue untouched.”
In lab studies, the therapy destroyed up to 92% of skin cancer cells and 50% of colorectal cancer cells in just 30 minutes, all without damaging nearby healthy skin cells. These results mark an exciting step toward safer, more precise cancer treatments.
Reducing Side Effects, Increasing Accessibility
Cancer remains one of the leading causes of death worldwide, and traditional treatments can take a serious toll on patients. Near-infrared photothermal therapy offers a safer path forward by using light to heat and kill cancer cells without drugs or surgery.
This LED-based approach could make such therapies more widely available. Traditional photothermal treatments require expensive lasers and complex lab setups. LEDs, by contrast, are low-cost and easy to scale, a major leap toward accessibility.
From the Lab to Real Lives
The team’s long-term vision includes developing portable medical devices that can deliver this light-based therapy directly to patients. For example, after surgery, a small LED patch could help eliminate any remaining cancer cells and lower the risk of recurrence, potentially even from home.
“We want this technology to reach patients everywhere,” said Artur Pinto, lead researcher at the University of Porto. “Our hope is to make cancer treatment more affordable, less invasive, and with far fewer side effects.”
A Promising Step for Breast Cancer Care
Building on their success, the researchers recently secured additional funding to adapt their LED and nanoflake system into an implantable device for breast cancer patients. This advancement could one day support breast cancer survivors by reducing recurrence risk in a safe, cost-effective way bringing us closer to personalized and pain-free treatment.
The collaboration, made possible by the UT Austin Portugal Program, continues to bridge engineering and medical innovation, driving forward new frontiers in cancer therapy.
Empowering Patients with Knowledge
At Breast Advocate, we believe that understanding the latest research empowers patients to make informed choices about their care. As breakthroughs like this continue to evolve, patients and clinicians alike gain more options for treatment options that protect both health and quality of life.
Sources:
- The University of Texas at Austin
- University of Porto
- ACS Nano Journal Publication
Your Guide to Breast Reconstruction Options After Breast Cancer
After breast cancer surgery, many women face decisions about breast reconstruction. There is no single “right” choice—only the option that best fits your needs and goals. Here are the main reconstruction options to consider.
Implant-Based Reconstruction
Implants are one of the most common methods. They can be filled with saline or silicone gel. Implant surgery is often shorter than flap reconstruction, and some women prefer implants because recovery may be faster. However, implants may need replacement over time, risks include infection, scarring, and implant-related complications.
Autologous Flap Reconstruction (Using Your Own Tissue)

Autologous reconstruction uses tissue from another part of your body. This method often looks and feels more natural than implants.
Common Flap Types
- DIEP flap – uses lower abdominal tissue, while preserving abdominal muscles.
- TRAM flap – also uses abdominal tissue, sometimes with some muscle.
- Latissimus dorsi flap – tissue is taken from the upper back.
- GAP / SGAP / IGAP flap – tissue comes from the buttocks.
- TUG flap – tissue comes from the inner thigh.
DIEP Flap vs. Tummy Tuck
The DIEP flap is sometimes mistaken for a tummy tuck. While both surgeries remove abdominal skin and fat, their goals are very different. The DIEP flap rebuilds the breast and preserves abdominal muscles. It involves reconnecting tiny blood vessels and may restore breast sensation. By contrast, a tummy tuck is a cosmetic procedure focused only on shaping the abdomen. Importantly, DIEP flap surgery is typically covered by insurance as part of breast reconstruction while a tummy tuck is considered cosmetic and usually is not covered.
(PRMA Enhance Breast Reconstruction Blog)
Flap surgeries take longer and require recovery from both the chest and donor site. However, many women appreciate the natural look, feel, and durability of flap options.
Flat Closure
Some women choose no reconstruction, flat closure means the chest is closed smoothly after mastectomy. This option avoids additional surgeries and often has faster recovery. Many women feel empowered with a flat closure choice. External breast prostheses remain an option if desired later.
Combination Reconstruction
Surgeons sometimes combine flaps with implants. This may provide both natural tissue coverage and implant support. Combination approaches can balance appearance, durability, and recovery.
Timing of Reconstruction
Reconstruction can happen immediately during mastectomy, It can even be delayed, even months or years later. Timing depends on cancer treatment, overall health, and personal preference.
Making the Decision
Every woman’s situation is unique. Each reconstruction choice has benefits and trade-offs.
Talking openly with your care team is essential.The Breast Advocate App is here to guide you with trusted information and decision tools. Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.
New Treatments from HER2 Breast Cancer Discovery
Researchers discovered that the HER2 gene plays a big role in breast cancer growth. This finding changed treatment options for many patients. Doctors noticed that some cancers grow slowly, while others spread quickly.In the 1980s, researchers found that certain genes might explain these differences.
The Discovery of HER2
Dr. Dennis Slamon and his team studied the HER2 gene. They discovered HER2 proteins appear in high levels in about 30% of breast cancers. High HER2 levels are linked to cancer growth, relapse, and lower survival. Researchers believed blocking HER2 could slow or stop tumor growth. Scientists tested antibodies to block HER2 proteins. The results were promising in lab studies and animal research. Genentech developed trastuzumab (Herceptin), a drug that targets HER2 in humans.
Trastuzumab Improves Survival
Clinical trials showed trastuzumab, combined with chemotherapy, slowed tumor growth. In 1998, results showed patients lived longer with this treatment. By 2006, trastuzumab was approved as a standard treatment for HER2-positive breast cancer. Survival rates improved by more than 30% for many patients.
New HER2-Targeted Therapies
However not all patients respond to trastuzumab. Some patients develop resistance to the drug. Researchers developed new treatments, like pertuzumab, Kadcyla, Tykerb, and Nerlynx. These therapies give more patients hope and better outcomes.
Ongoing Research
Scientists continue to create HER2-targeted treatments. The goal is to fight more cancers, improve survival, and reduce side effects.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.
New Animal Study Suggests Possible Link of Seed Oil Fatty Acid to Triple-Negative Breast Cancer Growth

A Key Discovery in Breast Cancer Research
An animal study performed in mice by Weill Cornell Medicine researchers suggests that linoleic acid could enhance triple-negative breast cancer growth. Linoleic acid is an omega-6 fatty acid found in seed oils and animal products. This study, published in Science, revealed a direct biological mechanism linking dietary fat to tumor growth in mice.
How Linoleic Acid Fuels Cancer Growth
The researchers discovered linoleic acid binds to a protein called FABP5. This binding activates mTORC1, a major tumor growth pathway. Triple-negative breast cancer cells contain high FABP5 levels, making them vulnerable to this effect.
Why Triple-Negative Breast Cancer Matters
Triple-negative breast cancer lacks hormone receptors targeted by existing therapies. It is one of the hardest subtypes to treat. While it is important to remember studies in animals don’t always translate well to humans, identifying dietary and drug-related targets could bring new hope to patients.
Dietary Fats and Cancer Risk
Omega-6 fatty acids are essential for health in small amounts. However, Western diets contain much higher levels of linoleic acid. This increase has sparked concerns about cancer risk for decades.
Personalized Nutrition and New Treatment Possibilities
The study suggests FABP5 may serve as a biomarker. Patients with high FABP5 could benefit from tailored nutritional guidance. New drugs may also target the FABP5-mTORC1 pathway directly.
Expanding the Research Beyond Breast Cancer
The same pathway was found in certain prostate cancers. Researchers believe FABP5-mTORC1 signaling could play roles in obesity and diabetes. More studies are needed to explore these broader connections.
Hope for Breast Cancer Patients
This discovery provides a link between diet and cancer biology. If studies in humans show similar results, this could open the door for more personalized, holistic breast cancer treatment strategies including tailored nutritional plans.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.
New 4-in-1 Drug Could Transform Weight Loss and Support Breast Cancer Patients

Scientists at Tufts University have created a new compound targeting four key hormones. The drug combines GLP-1, GIP, glucagon, and PYY for greater effectiveness. This innovation could match the 30% weight loss often seen with bariatric surgery.
Why This Matters for Breast Cancer Patients
Obesity is a major risk factor for breast cancer development and recurrence. Excess weight raises estrogen levels and fuels tumor growth. A safe, effective weight loss treatment could lower breast cancer risks significantly.
Current Drugs Have Serious Limitations
Medications like Ozempic and Wegovy often cause nausea and muscle loss. Many patients stop using them due to unpleasant side effects. Weight regain is common once treatment ends.
The Promise of the Tufts Compound
The new 4-in-1 therapy aims to overcome these issues. By hitting four hormone receptors, it provides stronger and more consistent results. Researchers hope this approach also preserves bone and muscle health.
Impact on Breast Cancer Survivors
Maintaining a healthy weight improves outcomes after breast cancer treatment. This drug could help survivors avoid obesity-linked recurrence risks. It may also reduce side effects linked to traditional weight loss drugs.
Looking Ahead
The Tufts team is preparing for future clinical trials. If successful, this drug could change obesity and cancer care worldwide. It represents hope for millions seeking lasting, safe weight loss.
At Breast Advocate, we’re committed to providing tools, education, and personalized decision support for those facing breast cancer.
Download the Breast Advocate App for trusted guidance and expert-backed resources, available 24/7.
Recognizing the Symptoms of Breast Cancer Early Can Save Lives

San Antonio, TX — Breast cancer remains one of the most common cancers worldwide, affecting millions of women and men each year.
But when caught early, breast cancer is highly treatable—making awareness of the signs and symptoms absolutely vital.
At the Breast Advocate App, we believe knowledge is power. Understanding what to look for can lead to earlier diagnosis, more treatment options, and better outcomes.
Most Common Symptoms of Breast Cancer
While not all breast changes mean cancer, certain signs should never be ignored.
The most common early symptom is a lump or mass in the breast or underarm.
“Most breast lumps are benign,” says Dr. Elisabeth Potter, breast reconstruction surgeon and medical advisor to Breast Advocate.
“But it’s essential to get any new lump checked out—especially if it feels firm, irregular, or painless.”
Other key symptoms include:
- Swelling of all or part of the breast
- Skin dimpling or puckering, sometimes resembling an orange peel
- Nipple retraction (pulling inward)
- Redness, scaliness, or thickening of the nipple or breast skin
- Nipple discharge, especially if clear or bloody and not associated with breastfeeding
- Pain in the breast or nipple area that doesn’t go away
Don’t Ignore Subtle Changes
Breast cancer can present differently in every person.
Sometimes, symptoms are not obvious or are mistaken for other conditions, like infections or cysts.
“Changes in breast shape, texture, or sensation—especially when new or persistent—should always be reported,” says Dr. Potter.
Men can also develop breast cancer, though it’s rare.
In men, signs often include a lump, nipple changes, or skin thickening near the chest.
When to Seek Medical Advice
If you notice any of the above symptoms, don’t wait.
See your doctor promptly for a clinical breast exam and possible imaging.
A mammogram, ultrasound, or MRI can help determine whether changes are benign or suspicious.
If needed, a biopsy may be performed to confirm a diagnosis.
Know Your Normal: Self-Awareness Saves Lives
Knowing what’s normal for your breasts is one of the best ways to catch changes early.
We encourage regular self-exams and breast self-awareness—not just during Breast Cancer Awareness Month.
“Check your breasts monthly and pay attention to how they look and feel,” says Dr. Potter.
“Even small changes are worth talking about.”
Not All Breast Cancers Have Symptoms
While many breast cancers cause noticeable signs, some are silent—especially in early stages.
That’s why routine screening is critical, even if you feel fine.
The U.S. Preventive Services Task Force now recommends mammograms every two years starting at age 40 for most women.
Those with a strong family history or genetic risk may need earlier or more frequent screening.
Final Thought from Breast Advocate
Being informed could save your life—or someone else’s.
If you notice something unusual, trust your instincts and speak with your care team.
At Breast Advocate, we’re committed to providing tools, education, and personalized decision support for those facing breast cancer.
Download the Breast Advocate App for trusted guidance and expert-backed resources, available 24/7.
Tirzepatide Shows Promise in Shrinking Breast Tumors

San Francisco, CA — A powerful weight loss drug used to treat obesity and diabetes may also help shrink breast tumors, researchers say.
The medication, tirzepatide—sold as Mounjaro and Zepbound—reduced tumor growth in obese mice, according to a study presented at ENDO 2025.
Researchers from the University of Michigan found that tirzepatide significantly reduced both body fat and breast tumor volume in test subjects.
Early Evidence Links Weight Loss Drug to Cancer Control
Obesity is a known risk factor for breast cancer and can worsen outcomes.
Tirzepatide activates GLP-1 and GIP receptors to reduce appetite and body weight.
“This is very early data, but encouraging,” said study author Amanda Kucinskas, B.S., a Ph.D. candidate at the university.
Inside the Study: Less Fat, Smaller Tumors
The study involved 16 mice fed a high-fat diet to induce obesity.
At 32 weeks old, the mice were given either tirzepatide or a placebo.
Over 16 weeks, mice on tirzepatide lost about 20% body weight and fat.
Researchers also noted significantly smaller tumors in the tirzepatide group.
Tumor size strongly correlated with overall body fat, including fat stored in the liver.
Could Tirzepatide Help Breast Cancer Patients?
Previous studies show that weight loss improves breast cancer outcomes.
However, achieving and maintaining weight loss can be very difficult.
These new findings suggest anti-obesity drugs might offer dual benefits:
Reducing body fat and possibly directly affecting tumor growth.
“We are investigating whether the drug has tumor-specific effects,” Kucinskas said.
More Research Underway
Further studies are ongoing in collaboration with Dr. Steve Hursting’s lab at the University of North Carolina.
Researchers aim to separate the weight loss effects from potential direct effects on cancer.
Tirzepatide’s role in cancer prevention or treatment is still under review.
What Patients Should Know
While human trials have not yet confirmed these effects, the findings are promising.
People with obesity-related breast cancer may benefit from discussing new options with doctors.
Tirzepatide may offer a new tool in the fight against breast cancer.
As always, treatment plans should be personalized and guided by medical teams.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.
New Discovery Paves the Way for More Effective Cancer Treatments by Strengthening Immune Cells
Evolutionary Mutation Could Explain Cancer’s Survival Tactics
A recent UC Davis Comprehensive Cancer Center study reveals an evolutionary mutation that weakens human immune cells against solid tumors. Published in Nature Communications, the research identifies a tiny genetic change that could impact cancer therapies.
FasL Protein and Its Role in Immune Defense
The immune protein Fas Ligand (FasL) plays a crucial role in triggering programmed cell death, or apoptosis, in cancer cells.
- FasL helps immune cells like CAR-T cells destroy tumor cells.
- However, this protein is vulnerable to attack by plasmin, an enzyme found in many solid tumors, including triple-negative breast cancer and ovarian cancer.
Unique Genetic Mutation in Humans
The study highlights a genetic difference in FasL between humans and non-human primates. This mutation makes FasL more susceptible to being disabled by plasmin.
- Humans have serine at position 153 of the protein, while non-human primates, like chimpanzees, have proline at the same position.
- Plasmin, often elevated in tumors, cleaves FasL, neutralizing its cancer-fighting abilities.
How Plasmin Weakens Immunotherapy
This vulnerability in FasL explains why CAR-T therapies and other immune-based treatments are more successful against blood cancers than solid tumors.
- Blood cancers don’t rely on plasmin for metastasis, while solid tumors such as ovarian cancer depend on plasmin to spread.
Blocking Plasmin Could Enhance Immunotherapy
The study also suggests that blocking plasmin or shielding FasL from cleavage could restore its function. This discovery could revolutionize cancer immunotherapy.
- Plasmin inhibitors or antibodies that protect FasL may increase immune responses in solid tumor patients.
Next Steps for Cancer Treatment
The findings open up new possibilities for improving immunotherapies, especially for plasmin-positive cancers. By targeting the tumor environment, scientists could enhance the effectiveness of current treatments.
The Role of Evolution in Human Cancer Susceptibility
As Jogender Tushir-Singh, senior author of the study, explains, the FasL mutation may have been beneficial for human brain development but became a trade-off in the context of cancer.
“Understanding these evolutionary changes will be key in personalizing and improving immunotherapy for difficult-to-treat cancers,” Tushir-Singh added.
A Step Toward Better Cancer Treatments
This groundbreaking study underscores the importance of understanding human evolution to improve cancer treatments. By tackling plasmin’s role in tumor growth, new therapies could offer hope to patients battling solid tumors.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.
Decline in Breast Cancer Deaths Among Young Women: Key Findings from New Study
From 2010 to 2020, breast cancer mortality among women aged 20-49 significantly declined across all subtypes and racial/ethnic groups. This was particularly evident starting in 2016, according to a recent analysis of data from the Surveillance, Epidemiology, and End Results (SEER) registry. The findings were presented at the American Association for Cancer Research (AACR) Annual Meeting 2025.
Declining Mortality Rates: A Positive Trend
Breast cancer death rates in young women have dropped sharply in the last decade. The study analyzed data from 11,661 breast cancer deaths among women aged 20-49.
- Incidence-based mortality decreased from 9.70 per 100,000 women in 2010 to 1.47/100,000 in 2020.
- The most significant decline was observed in the luminal A subtype, with a 32.88% drop in 2017.
These results show that the collective efforts in improving breast cancer treatment, screening, and care have had a profound impact.
Racial and Ethnic Disparities in Mortality
While mortality declined for all racial/ethnic groups, the trends varied significantly:
- Non-Hispanic Black women had the highest incidence-based mortality in both 2010 (16.56/100,000) and 2020 (3.41/100,000).
- Non-Hispanic White women experienced the lowest mortality rates in both years, with a drop from 9.18/100,000 to 1.16/100,000.
Marked declines for various groups began in different years, with the most pronounced drops starting in 2016 for non-Hispanic Black women.
Luminal A and Survival Rates
The study also revealed unexpected findings regarding luminal A breast cancer:
- Luminal A had the best 10-year survival rate among women aged 40-49, but among those aged 20-39, luminal Bhad a higher survival rate (84.2%) compared to luminal A (78.3%).
This suggests a more complex biology in younger women with luminal A, who may experience more aggressive tumors.
Role of Treatment Advances
Advances in precision medicine and CDK4/6 inhibitors have likely contributed to these positive trends. These treatments became more widely available in 2015-2016, particularly for hormone receptor-positive, HER2-negative cancers like luminal A.
“We’ve made great strides, but challenges remain, particularly for younger women and racial/ethnic minorities,” said Dr. Adetunji Toriola, the lead author of the study.
Focus on Future Research and Care
The study emphasizes the need for continued research into breast cancer’s molecular mechanisms, particularly in young women.
Key recommendations:
- Ongoing research on tumor biology and treatment responses.
- Increased access to population-based screening for women ages 40-49.
- Targeted screening for younger high-risk women.
- Advocating for equitable access to quality treatment and care.
A Call for Continued Progress
While the reduction in breast cancer mortality is a significant achievement, experts agree that there is still room for improvement, particularly in addressing disparities across racial and ethnic groups.
“We must continue to strive for greater progress in reducing breast cancer mortality and ensuring access to care for all women,” Dr. Toriola emphasized.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.

Genetic Factors Contribute to Breast Cancer Risk in South Africa, Study Reveals
A recent study published in Nature Communications has uncovered two genetic variants linked to breast cancer in black South African women. This breakthrough underscores the need for genomic research tailored to African populations, offering new insights into cancer risk.
First Genome-Wide Study on African Women
This genome-wide association study (GWAS) is the first to examine breast cancer genetics specifically in African women living on the continent. Researchers at the Sydney Brenner Institute for Molecular Bioscience (SBIMB) analyzed the genetic data of black South African women, aiming to uncover factors contributing to breast cancer risk.
GWAS is a powerful tool used to scan genomes across large populations, identifying genetic variations tied to diseases. This study represents a significant step in understanding breast cancer genetics in African populations, which have historically been underrepresented in global research.
Two Key Genetic Variants Discovered
The research team identified two major genetic signals linked to breast cancer, located near RAB27A and USP22 genes.
- RAB27A: A member of the RAS oncogene family, playing a role in cancer cell growth and tumor development.
- USP22: A gene involved in breast cancer cell activity, linked to poor prognosis in cancer patients.
These two variants were found to have a consistent presence in women with breast cancer, suggesting their significant role in disease development. This discovery provides fresh insight into breast cancer genetics for women of African ancestry.
Addressing Genomic Gaps in African Populations
Until now, most breast cancer genetic research has focused on European and Asian populations, with limited studies on African populations. This study is a critical step in addressing these gaps, providing new data on how genetic factors affect breast cancer risk in sub-Saharan Africa.
Current Risk Prediction Tools Inaccurate for Africans
The study also highlighted limitations in existing cancer risk prediction tools, such as the polygenic risk score (PRS). PRS, which has been developed primarily based on European genetic data, performed poorly in identifying breast cancer risk in South African women.
Dr. Jean-Tristan Brandenburg, another lead author, stated, “PRS was not effective for African populations, highlighting the need for tools developed specifically for African ancestry.”
Urgent Need for African-Focused Genomic Research
Breast cancer is the second most common cancer in South Africa and the most prevalent cancer in women globally. Genetic factors account for about 30% of breast cancer cases. This study emphasizes the urgent need for more genomic research rooted in African contexts.
Dr. Mahtaab Hayat, the lead author, said, “Our findings make a strong case for investing in genomic research that is focused on African populations. We can better understand disease risk and improve early detection and treatment for these women.”
Potential for New Cancer Treatments
The identification of these genetic variants offers the possibility for more targeted therapies. If further studies confirm the role of RAB27A and USP22, these genes could become important targets for new cancer treatments.
Professor Chris Mathew, senior investigator at SBIMB, noted, “Targeting these genes could help destroy cancer cells while sparing healthy tissue. This would improve cancer treatment outcomes significantly.”
Genomic Diversity in Africa
Africa holds the world’s most diverse genetic population. However, African populations have been significantly underrepresented in genomic research. Dr. Hayat highlighted that this study proves the vast potential for discovering new genetic risk factors, particularly for African populations.
“Our study shows that there are still new risk factors out there waiting to be discovered,” Dr. Hayat concluded. “With increased focus on African genomic research, more individuals can benefit from these discoveries.”
Conclusion
This study underscores the critical need for genomic research that reflects the genetic diversity of African populations. By improving our understanding of breast cancer genetics in South Africa, scientists can develop more accurate risk prediction tools and more effective treatments for diverse populations worldwide.
Stay informed and engaged with the latest advancements. Empower yourself with knowledge and make more informed decisions about your breast cancer treatment and care. Visit the Breast Advocate App website today and join us in the fight against breast cancer.