Financial Toxicity of Metastatic Breast Cancer On The Rise

The reality of financial strain for breast cancer patients is not new news. A recent study suggests the financial toxicity associated with living with metastatic breast cancer may more than double over the next decade.
A study from the UNC Center for Health Promotion and Disease Prevention predicts that annual costs associated with metastatic breast cancer among United States women will be close to $152.4 billion in 2030. The large increase is due to a rise in the estimated cases of metastatic disease among younger women.
What is the predicted cost of metastatic breast cancer?
The study used the most recent U.S. census data as well as statistics from the National Cancer Institute to estimate how the number of women affected by metastatic breast cancer will change by 2030. Their model estimates a 54.8% increase in metastatic breast cancer diagnoses among women aged 18-64. This would translate to a rise in cases from 158,997 women living with the disease in 2015, to 246,194 in 2030. When combining this estimated data with the predicted annual cost of treatment, the future annual cost of metastatic breast cancer could reach $152.4 billion in 2030.
Funding key to reducing the risk of breast cancer
The study authors are hopeful that these statistics will promote more funding for early detection campaigns, access to care, and new treatments to help cure breast cancer.
Education and access to care are vital to early detection and prompt treatment. There are also many lifestyle factors that patients can consider to reduce their overall risk for developing breast cancer:
- Limiting alcohol consumption
- Maintaining a healthy weight
- Staying physically active
- Breast feeding when possible
- Limiting postmenopausal hormone therapy
As always, it is always very important to follow up regularly with your healthcare team and schedule your annual breast cancer screening appointments as recommended.
BETting Against Brain Mets With Promising Combination Therapy
Breast cancer metastasis (also known as “stage IV”) occurs when cancer cells leave the breast and travel to other parts of the body in the bloodstream or via the lymphatic system. The most common sites of spread are the liver, brain, bones, and lungs. About 30% of women diagnosed with early-stage breast cancer will ultimately develop metastatic disease. About 55% of women with HER2-positive breast cancer will progress to stage 4. Metastasis greatly impacts long-term survival; for breast cancer patients whose cancer has metastasized to the brain, the life expectancy is only about six months.
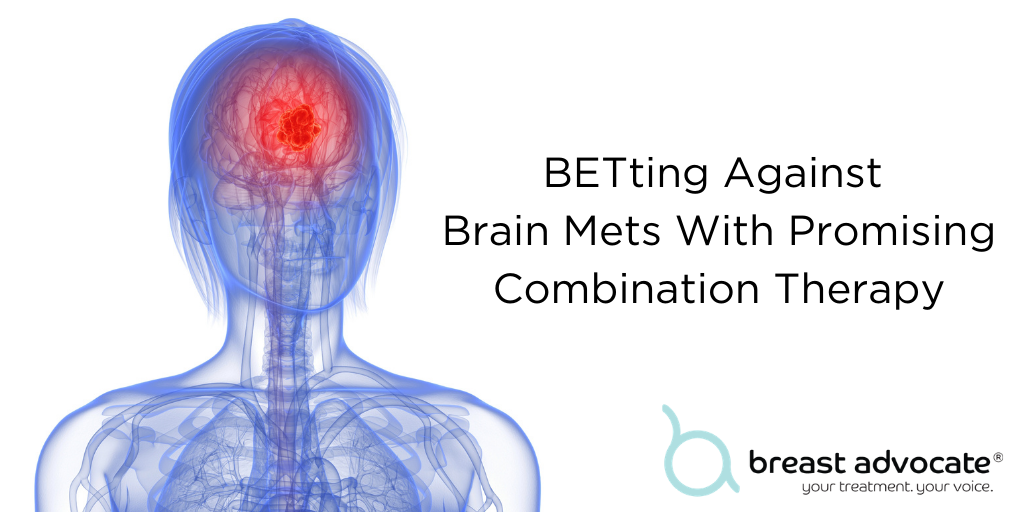
Brain metastases are difficult to reach and often are not susceptible to the same treatments as breast cancers in other parts of the body because they are blocked by the blood-brain barrier. However, a new study from Northwestern Medicine is reports some positive findings on a new treatment option.
A new combination therapy including a class of drug known as a BET inhibitor, greatly decreased the size of brain mets and increased survival in mice. About 75% of the mice who underwent this new treatment were cancer-free following the treatment. The BET inhibitor appears to sensitize breast cancer brain metastases to vinorelbine, a drug already approved by the FDA, demonstrating a potentially very promising therapeutic combination.
“The new combination therapy we identified can cross the blood-brain barrier,” said lead study author Dr. Maciej Lesniak, Northwestern Medicine chair of neurological surgery and professor of neurosurgery at Northwestern University Feinberg School of Medicine. “The therapy also targets brain metastases and significantly improves survival.”
The drug I-BET-762, used in combination with vinorelbine in the study, is only approved for trials by the FDA at this time.
FDA Approves New Drugs For Metastatic Breast Cancer

There was very hopeful news this week for many patients with metastatic (stage 4) breast cancer.
The FDA has approved the use of the drug Tukysa (tucatinib) in combination with chemotherapy (trastuzumab and capecitabine) for the treatment of locally advanced HER2+ breast cancer that can’t be removed with surgery, or for stage 4 disease (including brain metastasis) in patients who have already received one or more prior treatments. In trial studies, 33% of patients treated with Tucatinib in combination with chemotherapy did not see their cancer progress in the first year and 2-year survival after starting treatment was 44.9%.
Based on the favorable results of a recent phase 2 study, the FDA also announced a fast-tracked approval of the drug Trodelvy (sacituzumab govitecan-hziy). The drug is specifically for patients with relapsed or refractory metastatic triple-negative breast cancer who have received at least two prior therapies for metastatic disease. According to the manufacturer, the drug specifically targets the receptor that encourages cancer growth and spread in the body. The study for Trodelvy included 108 patients. More that half of patients with stage 4 disease who responded to the drug maintained their response for six months or more. Some patients also experienced reduced tumor sizes. Encouragingly, 16.7% of patients maintained their positive response to the drug for a year or more.
As always, before beginning any new therapy, it is important to fully discuss all the potential benefits and side effects with your medical oncologist.






